Medical PCB assembly is a crucial aspect of modern healthcare technology. PCBs, or printed circuit boards, are an integral part of medical devices such as pacemakers, insulin pumps, and diagnostic equipment. These devices require PCBs that are designed and assembled to the highest standards of quality and reliability to ensure the safety and well-being of patients.
The assembly of medical PCBs requires specialized expertise and equipment due to the stringent requirements of the medical industry. Medical PCBs must meet strict regulatory standards such as ISO 13485 and FDA regulations to ensure that they are safe and effective for use in medical devices. Additionally, medical PCBs must be designed to withstand harsh environments and extreme temperatures, as well as provide high levels of accuracy and precision.
In this article, we will explore the importance of medical PCB assembly and the challenges involved in designing and manufacturing PCBs for medical devices. We will also discuss the various techniques and technologies used in medical PCB assembly to ensure that these devices meet the highest standards of quality and reliability.
Overview of Medical PCB Assembly
Medical PCB assembly is a specialized process that involves the manufacturing of printed circuit boards (PCBs) for medical devices and equipment. These PCBs are used in various medical applications such as diagnostic equipment, monitoring devices, and implantable devices.
The assembly process of medical PCBs requires a high level of precision and expertise to ensure that the final product is safe, reliable, and meets all necessary regulatory standards. The components used in medical PCB assembly must also be carefully selected to ensure that they are suitable for use in medical devices.
One of the most critical aspects of medical PCB assembly is the quality control process. This process involves testing and inspecting the PCBs at various stages of the assembly process to ensure that they meet all necessary specifications. This includes testing for electrical conductivity, thermal management, and durability.
Medical PCB assembly also involves adhering to strict regulatory requirements, such as the FDA’s Quality System Regulation (QSR) and ISO 13485 certification. These regulations ensure that the PCBs are manufactured in a controlled environment and meet all necessary safety and quality standards.
In summary, medical PCB assembly is a specialized process that requires a high level of precision, expertise, and adherence to regulatory requirements. The quality control process is critical to ensuring that the final product is safe, reliable, and meets all necessary specifications.
Design Considerations

When designing a medical PCB assembly, there are several factors to consider to ensure that the final product is safe, reliable, and effective. Here are a few key design considerations to keep in mind:
Component Selection
One of the most critical aspects of PCB design is selecting the right components. When it comes to medical applications, this is especially important, as the wrong component choice can have serious consequences. Some factors to consider when selecting components for medical PCB assemblies include:
- Reliability: Components should be chosen for their reliability and long-term performance.
- Safety: Components must be safe for use in medical applications and comply with relevant regulations and standards.
- Size: Medical devices are often small and compact, so components should be chosen with size in mind.
- Cost: While cost is always a consideration, it should not be the primary factor in component selection for medical PCB assemblies.
Layout Design
The layout design of a PCB is also critical to its performance and reliability. When designing the layout for a medical PCB assembly, some important considerations include:
- Trace routing: Trace routing should be carefully planned to minimize noise and interference.
- Component placement: Components should be placed strategically to minimize the distance between them and reduce the risk of signal degradation.
- Thermal management: Medical devices often generate heat, so thermal management should be considered in the layout design to prevent overheating.
- Signal isolation: Medical devices require high levels of signal isolation to prevent interference and ensure accurate readings.
Signal Integrity
Finally, signal integrity is a crucial consideration when designing medical PCB assemblies. Signal integrity refers to the quality of the signals transmitted through the PCB, and it can be affected by a range of factors, including noise, interference, and signal loss. To ensure optimal signal integrity, designers should consider:
- Trace impedance: Trace impedance should be carefully controlled to minimize signal loss and maintain signal quality.
- Grounding: Proper grounding is essential to reduce noise and interference.
- EMI shielding: Medical devices must be shielded from electromagnetic interference (EMI) to prevent signal degradation.
By carefully considering these design factors, designers can create safe, reliable, and effective medical PCB assemblies that meet the unique needs of the medical industry.
Manufacturing Process
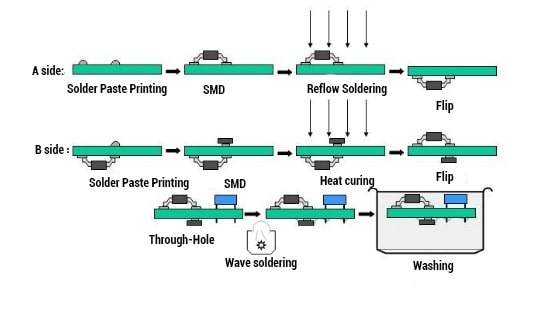
Solder Paste Application
The first step in the medical PCB assembly process is to apply solder paste to the board. Solder paste is a mixture of tiny metal balls and flux, which is a chemical that helps the solder flow and bond to the board. This paste is applied to the board using a stencil that is placed over the board and aligned with the pads where the components will be placed. The solder paste is then pushed through the stencil and onto the board using a squeegee or a machine. The stencil is removed, leaving the solder paste in place on the board.
Pick and Place
The next step is to place the components onto the board. This is done using a pick and place machine, which is a robotic device that picks up the components from a reel or tray and places them onto the board in the correct location. The machine uses a camera to ensure that each component is placed in the correct orientation and location. This process is repeated for each component until all of the parts have been placed on the board.
Reflow Soldering
The final step in the PCB assembly process is to solder the components to the board. This is done using a reflow oven, which heats the board and the solder paste until the solder melts and bonds the components to the board. The oven is programmed to heat the board to a specific temperature for a specific amount of time to ensure that the solder melts and flows correctly. Once the board has been heated and cooled, it is inspected to ensure that all of the components are securely attached and that there are no defects in the solder joints.
In summary, the manufacturing process for medical PCB assembly involves applying solder paste to the board, placing the components onto the board using a pick and place machine, and soldering the components to the board using a reflow oven. Each step in the process is critical to ensuring that the PCB is assembled correctly and functions properly.
Quality Control
When it comes to medical PCB assembly, quality control is of utmost importance. Here are the three main aspects of quality control in the assembly process:
Visual Inspection
Visual inspection is the first step in ensuring that the PCB assembly meets the required quality standards. This process involves a thorough examination of the PCB assembly for any defects, such as incorrect component placement, soldering defects, or damaged components. Any defects found are documented and corrected before moving on to the next step.
Functional Testing
Functional testing is the next step in ensuring that the PCB assembly is working as intended. This process involves testing the PCB assembly under various conditions to ensure that it meets the required performance specifications. The testing process can be automated or manual, depending on the complexity of the PCB assembly. Any issues found during testing are documented and corrected before moving on to the final step.
Traceability
Traceability is an essential aspect of quality control in medical PCB assembly. It involves tracking each component used in the assembly process, as well as the manufacturing process itself. This ensures that any issues can be traced back to their source and corrected quickly. Traceability also helps to ensure that the PCB assembly meets all regulatory requirements.
In summary, quality control is critical in medical PCB assembly. Visual inspection, functional testing, and traceability are the three main aspects of quality control in the assembly process. By following these steps, manufacturers can ensure that their PCB assemblies meet all required quality standards and regulatory requirements.
Regulatory Compliance
Ensuring regulatory compliance is a critical aspect of medical PCB assembly. There are several regulations and standards that must be met to ensure the safety and effectiveness of medical devices.
ISO 13485
ISO 13485 is an international standard that outlines the requirements for a quality management system specific to medical devices. This standard covers the entire life cycle of a medical device, from design and development to production, installation, and servicing. Compliance with ISO 13485 is a requirement for medical device manufacturers in many countries.
FDA Regulations
The US Food and Drug Administration (FDA) regulates medical devices sold in the United States. Medical device manufacturers must comply with FDA regulations to ensure the safety and effectiveness of their products. The FDA classifies medical devices into three categories based on the level of risk they pose to patients. Class I devices are considered low-risk, while Class III devices are considered high-risk and require the most stringent regulatory oversight.
CE Marking
CE marking is a requirement for medical devices sold in the European Union. This marking indicates that the device meets certain safety, health, and environmental protection standards. Medical device manufacturers must comply with the EU’s Medical Devices Regulation (MDR) to obtain CE marking.
In conclusion, regulatory compliance is a crucial aspect of medical PCB assembly. Compliance with ISO 13485, FDA regulations, and CE marking is necessary to ensure the safety and effectiveness of medical devices.

